Vertebrate gonad development and function
The gonad is essential for species propagation as it produces gametes and in vertebrate animals regulates sexual development. The vertebrate gonad is composed of the somatic support cells and the germ cells. Somatic cells function to: 1) regulate the development and maturation of gametes, 2) form the niche for the maintenance of the germline stem cells and 3) control sexual development by secreting sex hormones. Thus defects in development of the gonad result in disorders of sexual development and infertility.
The Draper lab uses the zebrafish as a model system to study vertebrate gonad formation and function. The zebrafish is an excellent model system as there are more similarities in the gonads of zebrafish and mammals than there are differences. For example, while the overall architectures of the zebrafish gonads differ from those of mammals, they share all of the same somatic cell types: Sertoli and Leydig cells in the testis and granulosa and theca cells in the ovary. In addition, it is known that in both mammals and teleost fish the interstitial cells (Leydig and theca) and the Sertoli and granulosa cells have a similar developmental origin. Finally, zebrafish have available a combination of excellent forward and reverse genetic tools, which together with the extra-uterine development of the animal, make it an ideal model for the study of gonad development. With appropriate reporter lines, the optical clarity of the animal during the time the gonad is developing makes it possible to observe the progression of gonad development in living animals. In addition, the recent advent of the TALEN and CRISPR/Cas9 genome editing systems make it now possible to rapidly produce loss-of-function alleles in any gene. We therefore have the technical ability to interrogate the role of any gene in gonad development and/or function.
Germline stem cells: We study mechanisms that regulate the development and function of germline stem cell (GSC) in zebrafish, with a main focus on female GSC. We have found that nanos3, which encodes an ortholog of the Drosophila germ cell-specific translational regulator Nanos, is required for the maintenance of GSC in the zebrafish ovary (Draper et al., 2007; Beer and Draper, 2013). We have also determined that nanos2 is expressed in germline stem cells in both males and females (Beer and Draper, 2013) and current work aims to determine the role of this gene in GSC specification and/or maintenance.
Somatic gonad development: Development of the vertebrate somatic gonad occurs in three phases: 1) initiation, 2) growth of a sexually indifferent gonad, and 3) sexual differentiation of the gonad. The mechanisms mediating the development of the somatic gonad during the transition from the sexually indifferent state to a sex-specified state are fairly well defined. By contrast, far less is known about the mechanisms that regulate the formation and growth of the undifferentiated gonad primordium. We have recently discovered that mutations in fgf24 affect this early stage of gonad development. Unlike most other genes previously defined to function in vertebrate gonad development, the fgf24 defect appears limited to the gonad compartment and does not disrupt other urogenital-derived organs.
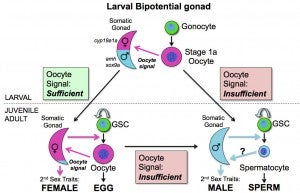
Sex determination and maintenance: The mechanism by which zebrafish determine their sex is largely not known. Several studies have discovered that germ cell, and in particular oocytes, play an active role in sex determination. During normal development, all animals initially produce oocytes between 15-25 days post-fertilization, and animals that cannot produce oocytes, due to mutation, develop exclusively as males. These and other results have led to the hypothesis that the oocytes produce a signal that stabilizes female development if a threshold level is produced. While zebrafish do not normally switch sex as adults, our lab has recently discovered that maintenance of the adult female sexual phenotype is an active process that, similar to primary sex determination, requires a signaling from oocytes, as reduction of oocyte numbers in an adult female can result in female-to-male sex reversal (Dranow et al., 2013). Our current research is focused on determining what signal(s) is produced by the germ cells and how it influences the developmental state of the somatic gonad.